Covid-19 Antigen Saliva Rapid Test -PCR Kit-In Stock
£9.91 ex.VAT
-
Antigen Test Kit Selco Hygiene & Healthcare 
Antigen Test Kit Pcr Selco Hygiene & Healthcare Antigen test using a saliva sample, so-called spit test, for personal use
- Easy to use, with pre-filled sample tubes
- With special approval from the BfArM
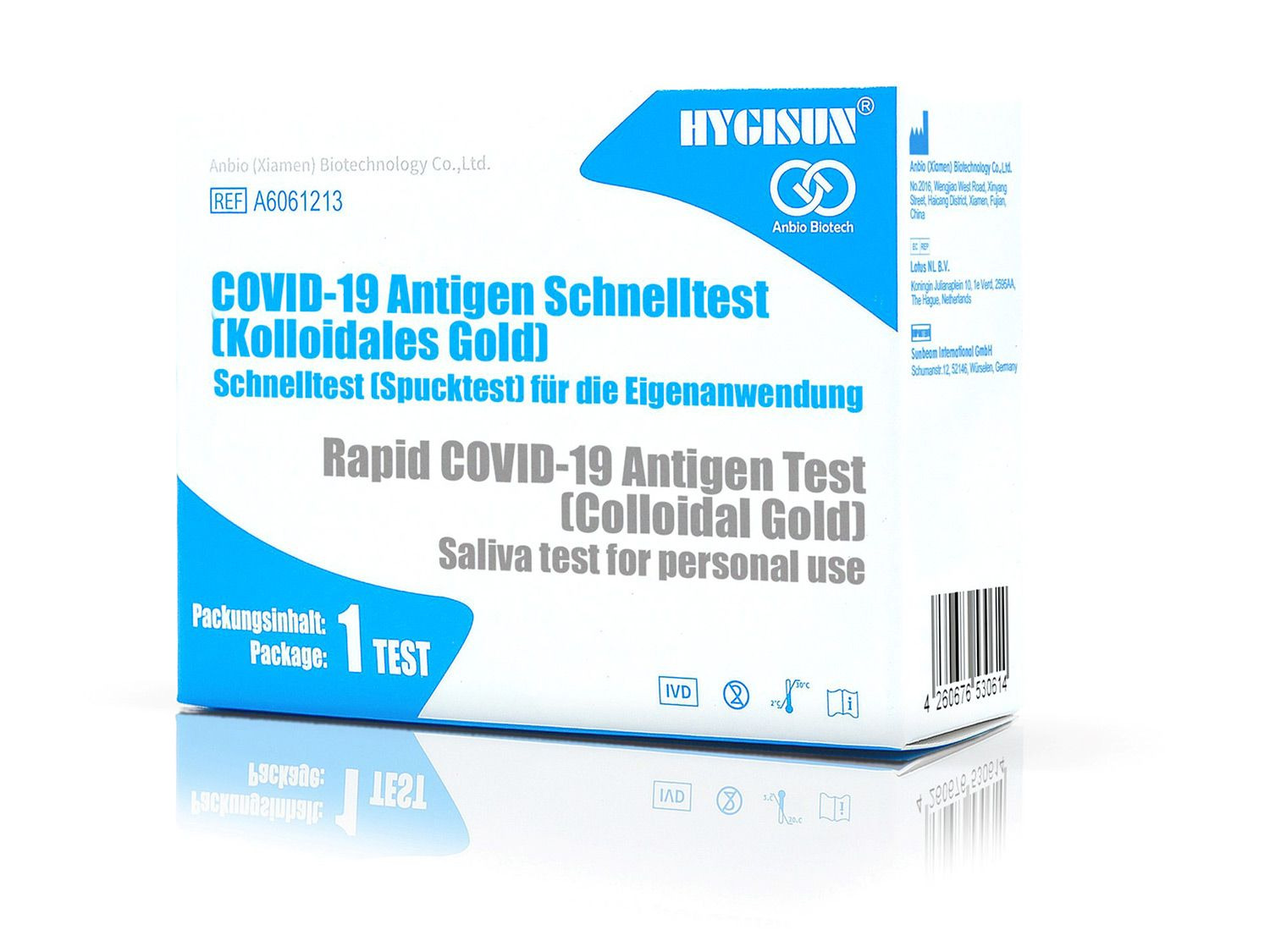
HYGISUN Covid-19 rapid test for laypeople
The HYGISUN COVID-19 antigen rapid test (colloidal gold) is an antigen test approved by the BfArM in accordance with Section 11 (1) of the Medical Devices Act (MPG) for self-use by laypeople. The file number of the special approval is: 5640-S-058/21.
The lay test manages completely without the nose and / or throat swab, which is usually perceived as very uncomfortable.
The HYGISUN COVID-19 antigen rapid test (colloidal gold) relies on the new test method in which the sample is simply taken from a saliva discharge, known colloquially as the “spit test”. This release of saliva enables a qualitative in vitro determination of the novel coronavirus antigen.
Sensitivity & Specificity
The result is already visible after 15-20 minutes. The HYGISUN COVID-19 Antigen Rapid Test (Colloidal Gold) thus serves perfectly as a rapid diagnosis. The test has a sensitivity of 98.19% and a specificity of 100% .
Related products
Best Buys
Best Buys

















